Synthetic Control over Magnetic Moment and Exchange Bias in All-Oxide Materials Encapsulated within a Spherical Protein Cage
Ferritin had previously been shown to be an excellent reaction vessel for controlled deposition of inorganic nanoparticles of both iron and cobalt oxides. Here we demonstrate that using ferritin under mild biomimetic reaction conditions, we can control mixed mineralization reactions of cobalt and iron oxides that lead to composite materials with tailored magnetic properties. Specifically, by modifying the synthesis conditions, we can generate phase segregated oxide particles of Fe3O4 and Co3O4 where exchange coupling at the interface between ferromagnetic and antiferromagnetic materials can introduce additional magnetic anisotropy effects leading to magnetic order at higher temperatures while maintaining small particle size.
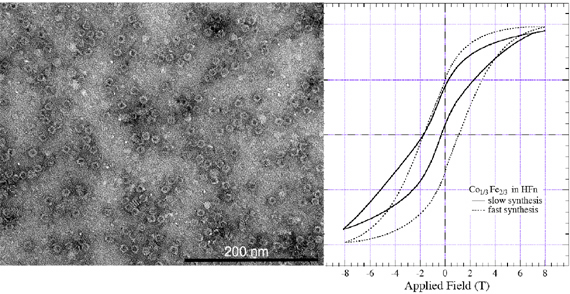
The products of the mineralization reactions were characterized by TEM and showed clear electron dense cores of a size consistent with the interior diameter of the protein cage. The particle sizes were found to be 6.8 – 7.4 nm. When negatively stained with uranyl acetate, the intact protein cage was clearly visible as a white ‘halo’ surrounding the metal oxide cores on the interior surface (figure below). Selected area electron diffraction experiments revealed d-spacings that were consistent with both the spinel phase of cobalt oxide (Co3O4) and the inverse spinel magnetite (Fe3O4), but excludes cobalt monoxide (CoO) as a major contributor. Hysteresis loops for sample synthesized under slow and fast conditions showed very different exchange bias (figure below).
In order to verify the cobalt oxide and iron oxide components of the electron diffraction, x-ray absorption spectroscopy (XAS) was performed to look at each component of the oxide in an element specific manner for comparison with reference XAS spectra. In each case, the collected Fe L3 and L2 edges spectra were consistent with Fe in a local environment of CoxFe3-xO4 (x = 0 – 0.33) across all dopant levels.
The Co L3 and L2 edges for a Co/Fe ratio of 0.33 yielded spectra whose shapes were dependent on the synthesis time. The Co was incorporated into each nanoparticle in two competing phases, Co3O4 or CoFe2O4 (where there is a clear spectral difference for Co in these two structures as demonstrated in the bottom XAS spectra). When the synthesis time was fast (5 minutes) the spectra exhibited lineshapes consistent with nanoparticles with Co in two distinct phases, Co3O4 and CoFe2O4. A comparison of the measured spectra with a normalized sum of the two reference spectra was consistent with a nanoparticle composed of 85% CoFe2O4 and 15% of Co3O4 (top XAS spectra ). When the synthesis time was extended to 30 minutes (slow) the observed spectra had undergone a dramatic change. This spectrum could be accounted for by summing standard spectra of CoFe2O4 and Co3O4 with a weighting of 25% and 85%, respectively (middle XAS spectra).
The Fe spectra are clear indicators that the Fe remains in the inverse spinel structure type of Fe3O4 whereas, with increasing Co concentration, the Co XAS spectra indicates that the Co either occupies Fe2+ site to give a material whose composition is CoxFe3-xO4 (x=0 – 0.33) or shifts toward Co(III) through the formation of Co3O4 depending on synthesis time. This valance variation clearly indicates that the Co is not fully incorporated into the iron oxide host, but rather is formed as a second antiferromagnetic phase, which can lead to a substantial magnetic exchange bias of the ferrimagnetic CoxFe3-xO4 oxide by the antiferromagnetic Co3O4 in the case of a slow synthesis.
The enhancement in the magnetic anisotropy, and corresponding increase in blocking temperature, was due to the introduction of Co into the synthetic reaction, and it was hypothesized that this phenomenon was a consequence of Co being incorporated into the Fe3O4 lattice. The decreasing trend in TB observed after 10% Co/Fe results from the formation of phase segregated oxides (Fe and Co) supported by the dramatic increase in exchange bias at 33% Co/Fe which resulted from the interaction between the ferrimagnetic (Fe3O4) and antiferromagnetic (Co3O4) particles within the protein cage. When the synthesis time was varied from 5 to 30 minutes, the blocking temperature showed only minor changes (50 K at 5 minute synthesis and 60 K for 30 minutes synthesis) and only a small change in the coercive field at 5 K (8.3 vs 7.8 kOe).
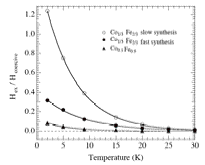
The temperature dependence of the exchange bias of 10% and 33% Co/Fe (figure at right) is consistent with the view that the particles are phase separated between a ferrimagnetic iron oxide particle in direct contact with an antiferromagnetic cobalt oxide which is the source of the exchange bias. In each case there is a rapid decrease in the Hex/Hc ratio as the temperature is increased, and the exchange bias disappeared at 30K, near the Néel temperature of Co3O4. The exchange bias was highest in the particles with a 33% Co/Fe loading ratio and synthesized slowly over a 30 min. period, as expected, since there is more antiferromagnetic Co3O4 present to bias the ferrimagnetic Fe3O4 core.
